How Does HFIP Enhance LC-MS Oligonucleotide Analysis?
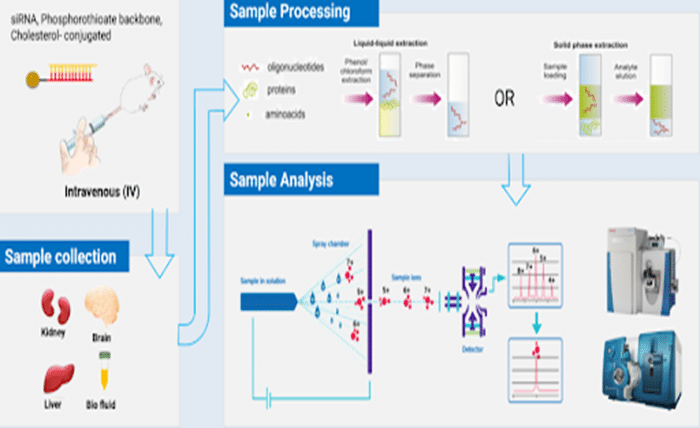
Hexafluoroisopropanol (HFIP) has become a valuable additive in liquid chromatography-mass spectrometry (LC-MS) for oligonucleotide analysis. This article explores how HFIP enhances the efficiency of LC-MS in analyzing oligonucleotides, improving ionization efficiency, and minimizing metal adduct formation. By understanding the benefits and practical uses of HFIP, researchers and analysts can achieve more accurate results in their oligonucleotide studies. As LC-MS continues to grow as a preferred analytical method in biotechnology and pharmaceutical labs, the role of HFIP is becoming even more critical.
Understanding HFIP in LC-MS
What is Hexafluoroisopropanol (HFIP)?
Hexafluoroisopropanol (HFIP) is a solvent with a unique chemical structure. It is a fluorinated alcohol, known for its volatility, low viscosity, and ability to form hydrogen bonds. These properties make HFIP an effective solvent in various chemical and analytical applications, particularly in enhancing the performance of LC-MS systems. Its polar nature also helps to solubilize nucleic acids and polar analytes that would otherwise be poorly retained on reversed-phase columns.
Role of HFIP in LC-MS
In LC-MS, HFIP serves as an ion-pairing agent and an organic modifier. Its primary role is to improve analytical performance when analyzing oligonucleotides. HFIP facilitates better ionization, leading to higher sensitivity in mass spectrometry. Additionally, it helps suppress the formation of metal adducts, which can otherwise complicate the interpretation of mass spectra. Because oligonucleotides contain phosphate groups, they easily bind with metal ions such as sodium or potassium, which makes accurate detection challenging. HFIP interrupts this binding process by forming more stable complexes that do not interfere with ionization.
Benefits of Using HFIP in Oligonucleotide Analysis
Enhanced Ionization Efficiency
One of the significant advantages of using HFIP in LC-MS is enhanced ionization efficiency. HFIP’s chemical structure enables it to reduce the surface tension of the solution, enhancing droplet formation during the electrospray process. This results in more efficient ionization of the oligonucleotides, significantly boosting the overall sensitivity and detection limits of the LC-MS system. In many studies, signal intensities have shown a marked increase when HFIP is present, especially in negative ion mode, where oligonucleotides are typically analyzed.
Reduction of Metal Adduct Formation
Metal adducts can interfere with the accurate analysis of oligonucleotides by creating additional peaks in the mass spectra. HFIP effectively binds to metal ions, preventing them from forming adducts with oligonucleotides. This reduction in metal adduct formation leads to cleaner spectra and more straightforward interpretation, allowing researchers to obtain more reliable data. Cleaner spectra also mean fewer issues with deconvolution software and more consistent quantitation results.
Improved Chromatographic Resolution
Another notable benefit of HFIP is its ability to improve chromatographic resolution. HFIP acts to stabilize the oligonucleotides in the liquid phase, leading to sharper and more distinct peaks in the chromatographic separation process. Improved resolution ensures better separation of oligonucleotide sequences, which is crucial for accurate identification and quantification in LC-MS analyses. This advantage becomes even more evident in the analysis of complex oligo mixtures or modified sequences, where small differences in base composition or chemical structure need clear resolution.
Practical Considerations for HFIP Use
Optimal Concentration Levels
Determining the optimal concentration of HFIP is essential for maximizing its benefits. Typically, concentrations between 0.1% and 1% (v/v) are used, depending on the specific requirements of the analysis. It is important to fine-tune the HFIP concentration to balance improved ionization efficiency and the suppression of metal adducts without negatively impacting chromatographic performance. Overuse of HFIP can increase baseline noise or interfere with detector response, so method development should always include optimization studies.
Compatibility with Ion-Pairing Reagents
HFIP is often used alongside other ion-pairing reagents to enhance oligonucleotide analysis. Commonly paired with triethylamine (TEA) or hexylamine, HFIP ensures better separation and peak shapes. Ensuring compatibility between HFIP and these reagents is crucial for achieving optimal results. A preliminary compatibility check can help in developing effective methods for comprehensive oligonucleotide analysis. Some labs have even developed proprietary buffer systems combining HFIP and amines to tailor their performance for specific oligonucleotide lengths or chemistries.
Case Studies and Applications
Comparative Analysis with Other Modifiers
Case studies comparing HFIP with other commonly used modifiers highlight its superior performance. Researchers have noted significantly improved ionization efficiency and reduced metal adduct formation when using HFIP compared to alternatives such as methanol or acetonitrile. In direct comparisons, HFIP-TEA mobile phases have shown higher sensitivity and lower noise for oligo detection than traditional methanol-based buffers, especially in high-throughput genomic workflows.
Real-World Applications
HFIP has found applications in various fields, including drug development and genetic research. For instance, in antisense oligonucleotide (ASO) studies, HFIP’s use in LC-MS has led to better sensitivity and specificity, facilitating the development of more effective therapies and diagnostic tools. Researchers studying mRNA, CRISPR components, and aptamers have also adopted HFIP-enhanced methods to improve the quality of their data. Its application extends to both discovery and quality control, making it a versatile tool in modern bioanalysis.
Conclusion
Hexafluoroisopropanol (HFIP) is an invaluable additive in review lc-ms oligonucleotide analysis, offering enhanced ionization efficiency, reduced metal adduct formation, and improved chromatographic resolution. By optimizing HFIP concentration and ensuring compatibility with other reagents, researchers can significantly improve the accuracy and reliability of their analytical results. The incorporation of HFIP in oligonucleotide analysis represents a significant advancement in the field, promising to streamline and enhance various research and development processes. As the demand for high-sensitivity, high-specificity bioanalytical techniques grows, HFIP will likely remain a cornerstone in the LC-MS analysis of oligonucleotides.




